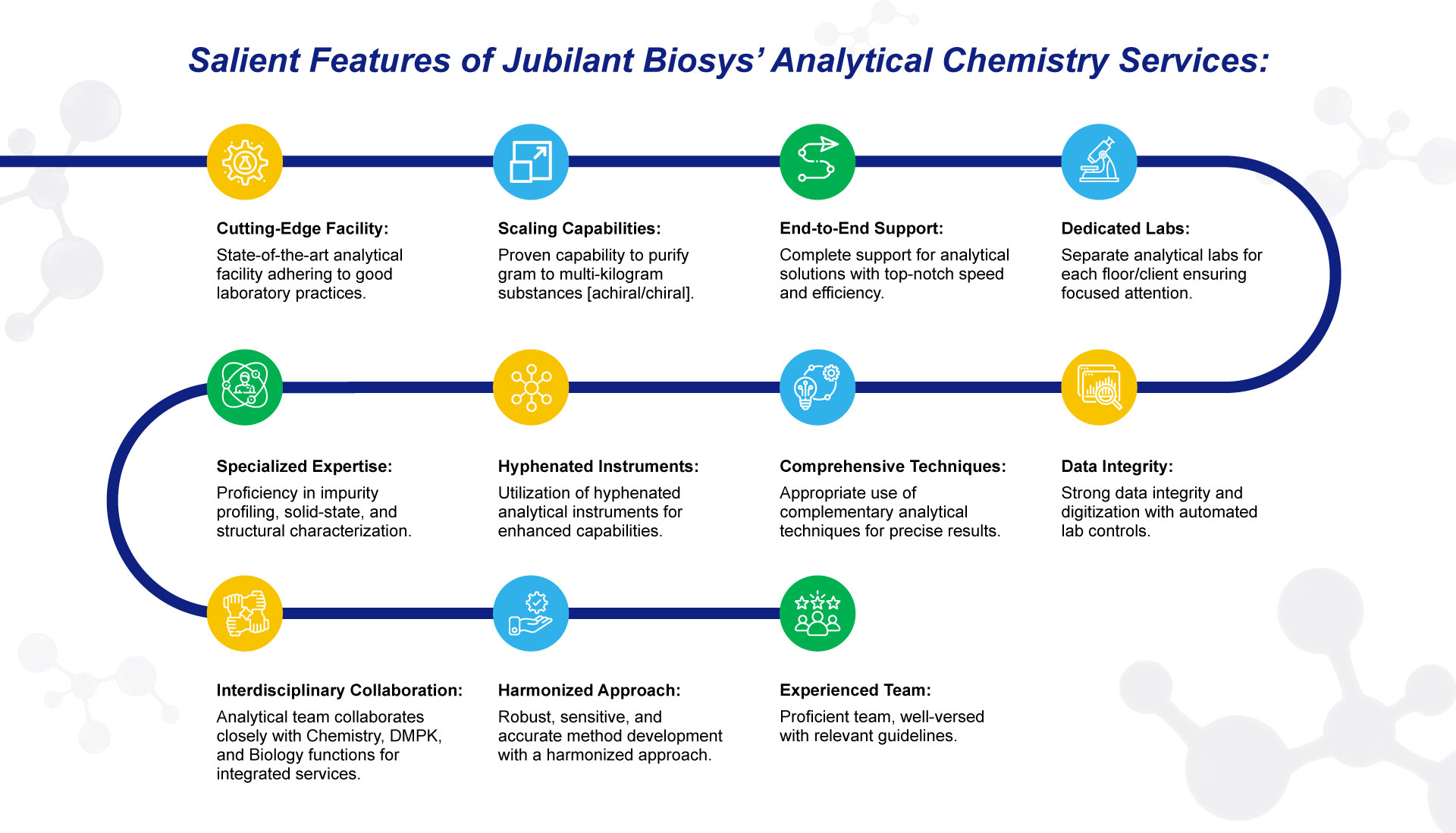
The analytical team provides support to discovery and process chemistry groups as an integrated service or a stand-alone option for our customers. Our unique approach allows us to identify and evaluate the chemical in our analytical testing laboratories rapidly and effectively communicate the resulting data to help our customers understand the product’s chemistry.
Purification
Structure Elucidation

Solid State Characterization
Physicochemical Study
Impurity Profiling
Analytical Method Development/Validation for CMC
Infrastructure (Analytical Instruments) Details & Capabilities