Pioneering Excellence in Integrated Drug Discovery
Jubilant Biosys offers a comprehensive, streamlined and integrated drug discovery process designed to achieve rapid milestones in the discovery of novel therapeutics. Our services under Integrated Drug Discovery span Target Validation (TV), Hit Identification (HI) to Lead Optimization (LO) and Pre-Clinical Candidate Selection (PCC) across multiple therapeutic areas including but not limited to oncology, CNS, metabolic disorders, pain & inflammation.
With well enabled capabilities in oncology, metabolic disorders, CNS, pain and inflammation Jubilant, as an integrated discovery service provider has rapidly emerged as a leading collaborator for biotechnology and pharmaceutical companies worldwide.
Jubilant’s expertise in discovery informatics, computational chemistry / molecular modelling, medicinal chemistry, structural biology, in-vitro pharmacology, DMPK, in-vivo pharmacology and toxicology span over 20 years of successful delivery in first in class and best in class targets.
We have expertise in multiple target classes including kinases/non-kinases, enzymes & non-enzymes, GPCRs, orphan receptors, transporters and others. We strive to leverage advanced technologies and expertise to foster speedy execution and deliver promising drug candidates.
Jubilant Biosys Limited Drug Discovery at a Glance
- 400+ people working on drug discovery at Bangalore.
- Demonstrated expertise: ~85 programs successfully delivered across multiple therapeutic areas.
Oncology
30+ Programs

Metabolic Disorder
15+ Programs
CNS
15+ Programs
Pain & Inflammation (P&I)
20+ Programs
Integrated Drug Discovery Services
Pre-Clinical Workflow
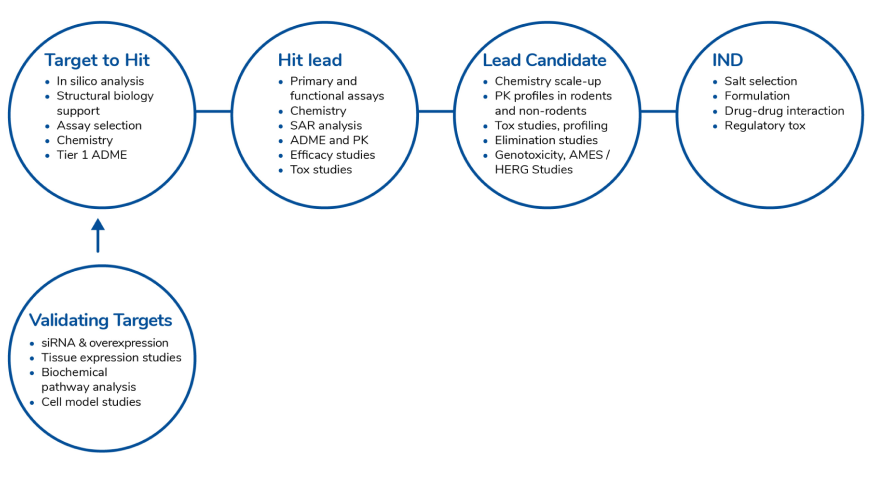
Target Validation
Jubilant Biosys’ target discovery group has a proven track record of validating disease-modifying targets across a range of target classes and therapeutic domains and successfully delivered numerous novel, validated drug targets.
Target validation represents a crucial initial stage in the development of a new drug, usually taking between 2 to 6 months to finalize. This vital process utilizes various techniques to show that the drug’s impact on the target can provide therapeutic advantages while maintaining an acceptable safety margin.
Comprehensive and early target validation is essential in establishing a strong link between target manipulation and its effectiveness in treating the disease, which greatly enhances the likelihood of success in clinical trials. Once a target reaches a satisfactory level of validation and connection to the disease, the project progresses to the hit identification phase.
HIT Identification
Hit identification is the process of identifying and delivering a compound with confirmed activity against a biological target and reproduces this activity when retested; hit finding typically occurs after target identification and validation, and before hit-to-lead and lead optimization studies.
The quality and properties of the hit series will delineate the chance of success at progressing rapidly into developable preclinical drug candidate.
Jubilant Biosys uses a variety of methods to identify high-quality, validated hits for known and novel targets, including literature searches, CADD-based de novo design, virtual screening such as molecular docking and molecular dynamics simulations, or fragment-based drug discovery (FBDD) and medium throughput screening.
- We expedite hit identification, focusing on protein structure or known ligands. We have consistently delivered hits against challenging protein classes using these technologies.
- Our broad ranging biophysics screening capabilities and crystallography know-how, will propel your fragment-based hits to potent leads.
When a suitable Hit is selected, it is engaged into several iterative DMTA (Design-Make-Test-Analyze) cycles in order to improve its physicochemical and ADME properties, potency, and selectivity to become a Lead.
Experienced hit identification team at Jubilant Biosys specializes in evaluating early-stage programs and working with you to design the best hit ID strategy for successful progression.
A Customized Approach
Jubilant operates on a customized approach offering innovative solutions across the drug discovery value chain to the biotechnology and pharmaceutical industry. To suit each client’s requirements in the best possible manner, we offer flexibility through the following business models:
- Integrated Drug Discovery Programs
- Milestone and Hybrid Models
- Full Time Equivalent (FTE) based
- Fee For Service (FFS) based
Jubilant collaborates with the world’s leading pharmaceutical and biotech companies, academic institutions and research foundations. Innovative and rigorous science, excellence in execution, and absolute integrity, combined with flexible business models has enabled Jubilant to deliver valuable outcomes in a relatively short period of time. The hallmark of Jubilant’s collaborative model is the creation of value to partners.
Various stages of Integrated Drug Discovery


Integrated
Drug Discovery
Workflow
Integrated Drug Discovery (IDD) SolutionsOffered by Jubilant Biosys

Target to Lead Generation
- Protein production, Crystallography, SPR platform for screening
- Fragment identification and Screening
- Medicinal & Computational Chemistry approaches
- In-vitro Screening and Profiling
- Early ADMET & PK
- In-vivo Pharmacology

Lead Optimisation
- Medicinal Chemistry
- DMPK
- SBDD (Co-crystallisation, Computational Modelling)
- Target Engagement & Disease Models, Safety Profile
- Pre-formulation

Candidate Selection (IND)
- Process Development (Including Flow Chemistry), Scale-up & GMP API Supply
- Genotox (Non-GLP & GLP Toxicology)
- D2M Predictions (WinNonlin)

API Custom Scale up
- GMP API scale- up to MT
- OEB Class 1-3 API Potency
Why Jubilant Biosys Limited for Integrated Drug Discovery?
- Successfully delivered 85+ IDD programs across multiple therapeutic areas.
- Preferred collaborator for biotech & pharma companies worldwide for the past two decades.
- Experienced team comprising scientific professionals, with Masters to PhD ratio of 6:1.
- PhDs with overseas exposure, having worked at premier institutions and organisations.
- Seamless, integrated approach to drug discovery.
- Medicinal chemistry expertise across multiple therapeutic areas and intervention strategies.
- De-novo assay development & validation capabilities; screening support and in vivo model development and validation.
- Timely ADMET support.
- Updated technologies & customised approach for developing innovative solutions.
- Best practices in documentation.
- Reliable quality solutions & & ISO 9001:2015, ISO14001:2015, ISO45001:2018 certified.
- End-to-end CRDMO solutions beyond IDD.