Jubilant Biosys has highly experienced team of analytical specialists to ensure all the analytical needs are met. This includes methods to support raw material release, in process/intermediate testing and final product analysis.
- All methods are developed with cGMP quality and are suitable for validation at the appropriate phase.
- We follow relevant ICH guidelines such as ICH Q2 (R1) or Pharmacopoeial methods/compendia guidance in combination with client-specific protocols.
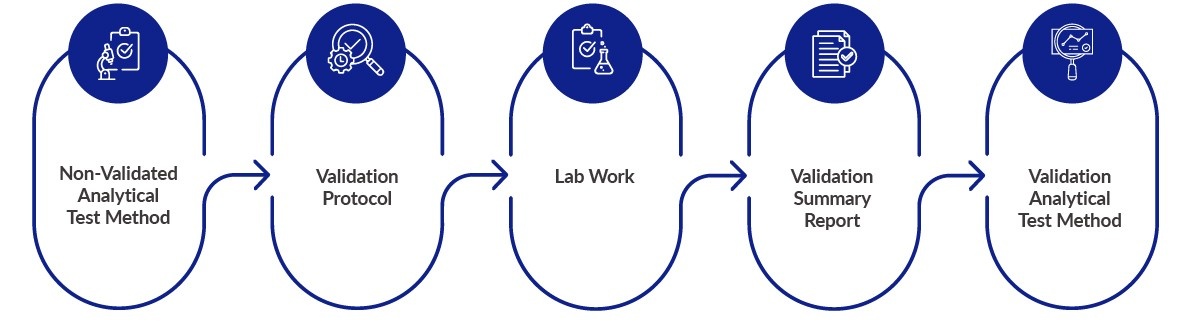
- Analytical method validation (a protocol-guided activity) ensures that a test procedure is accurate, reproducible, and sensitive within a specified range based on the application of the method.
- Through close evaluation of performance characteristics against pre-approved acceptance criteria, analytical method validation assesses an analytical method to demonstrate its suitability for the intended purpose.
Method Validation Process